
1.
Property Analysis, water mass analysis, isopycnal analysis
Two
objectives of studying the general circulation are to
determine the velocity structure and also the pathways for
water parcels. We are also interested in the fluxes of various
properties. For physical oceanography and climate, heat and
freshwater fluxes are of interest. For climate and
biogeochemical cycles, fluxes of other properties such as
carbon and nutrients are of interest.
Most
of our knowledge of the circulation is somewhat indirect,
using the geostrophic method to determine velocity referenced
to a known velocity pattern at some depth. If the reference
velocity pattern is not known well, then we must deduce it.
Deduction of the absolute velocity field is based on all of
the information that we can bring to bear. This includes
identifying sources of waters, by their contrasting
properties, and determining which direction they appear to
spread on average.
Water
properties are used to trace parcels over great distances.
Over these distances, parcels mix with waters of other
properties. It is assumed that mixing is easier along
isentropic (isopycnal) surfaces than across them and certainly
changes in T/S characteristics do often compensate (so that
density remains unchanged). However, it is clear from
distributions of some properties that of course there is
mixing both along and across isopycnals (isopycnal and
diapycnal mixing). This tracing of waters is useful (in
conjunction with the relative geostrophic flow calculations
that can be made from the observed density field), in order to
describe the average general ocean circulation.
We
use the concept of water
masses
as a convenient way to tag the basic source waters. The
definition of a "water mass" is somewhat vague, but is in
the sense of "cores" of high or low properties, such as
salinity or oxygen, in the vertical and along isopycnal
surfaces. A range of densities (depths) is usually
considered for a given water mass. Water mass definitions
may change as a layer is followed from one basin or ocean to
another, particularly if the trans-basin exchange involves
mixing.
Traditionally,
water mass analysis was based on plotting various properties
against each other, and attempting to explain the observed
distributions of properties as a result of mixing between the
identified "sources". However, point sources of waters occur
only in relatively few regions, and in general "source" waters
have a range of properties. The sources are almost always
surface waters, or near-surface waters that are created by,
say, air-sea interaction, brine rejection, or flow over and
through a narrow passage/sill.
The
most commonly used property-property displays are (a)
potential temperature vs. salinity, and (b) properties along
isopycnal surfaces. Figure.
Potential temperature versus salinity along 20 and 25W in
the Atlantic Ocean, from Iceland across the equator to South
Georgia Island.
Blue - equator to Iceland. Red - equator to about 30S. Green - 30S to South Georgia Island. The Atlantic 25W meridional potential temperature and salinity sections were already shown.
Blue - equator to Iceland. Red - equator to about 30S. Green - 30S to South Georgia Island. The Atlantic 25W meridional potential temperature and salinity sections were already shown.
2.
Tracers
Seawater
properties are valuable tools for tracing water parcels,
because water mass formation processes imprint distinct
properties on the water parcels. They are of most use when the
sources and sinks of one property compared with another
differ. Some tracers are biogenic and hence non-conservative.
These include oxygen and the various nutrients, all discussed
very briefly here. Some useful tracers are inert but with
time-dependent inputs, such as chlorofluorocarbons (CFCs).
Some useful tracers have decay times and decay products, which
can serve as a useful measure of age, such as bomb tritium and
helium. The latter are referred to as transient tracers, and
are not discussed here.
8.1.
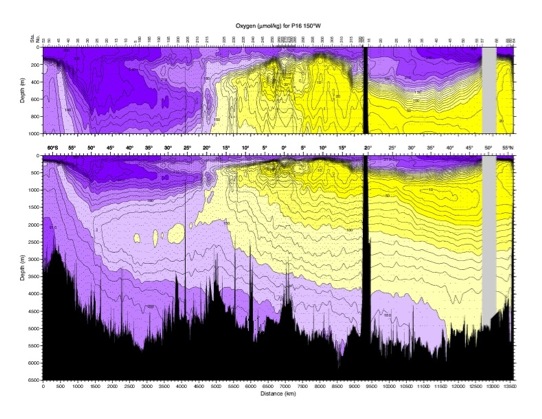
Oxygen.
Non-conservative tracer. The atmosphere is the primary
source of oxygen in the ocean and surface waters are usually
close to saturation. Per cent saturation of oxygen depends
strongly on temperature. Cold water holds more oxygen. Below
the surface, oxygen concentration decays as a result of
consumption by organisms and by oxidation of detritus
(marine snow). Therefore, oxygen
content decreases with age,
so it can be used in a rough way to date the water,
particularly at depth where consumption is very small.
However, it is not a precise age tracer because the
consumption rate is not a constant. Also, since waters of
different oxygen content mix, the age is not simply related
to concentration. However, low values of oxygen, such as
those found in the North Pacific deep and intermediate
layers, are an indication of waters that have been away from
the sea surface for a long time. In the Black Sea there is
no oxygen (anoxic) but hydrogen sulphide is present instead
(from reduction of sulphate by bacteria). This indicates
very stagnant deep waters.
8.2.
Nitrate and phosphate:
Both non-conservative. Nitrate and phosphate are completely
depleted in surface waters in the subtropical regions where
there is net downwelling from the surface and hence no
subsurface source of nutrients. In upwelling regions there
is measurable nitrate/phosphate in the surface waters.
Nitrogen is present in sea water in dissolved N2 gas,
nitrite, ammonia, and nitrate, as well as in organic forms.
As water leaves the sea surface, particularly the euphotic
zone, productivity is limited by sunlight and nutrients are
"regenerated". That is, the marine snow is decomposed by
bacteria and produces nitrate and phosphate. Nitrate
and phosphate thus increase with the age of the water.
Vertical sections and maps of nitrate and phosphate appear
nearly as mirror images of oxygen, but there are important
differences in their patterns, particularly in the upper
1000 meters.
Nitrate/oxygen
and phosphate/oxygen combinations - nearly conservative
tracers. Nitrate/oxygen and phosphate/oxygen are present in
seawater in nearly constant proportions, given by the Redfield
ratio. The Redfield ratio is C:NO3:PO4:O2 = 105:15:1:135.
There are small variations in this ratio, with particularly
large deviations near the sea surface. Because of the near
constancy of this ratio, a combination of nitrate and oxygen
and of phosphate and oxygen is a nearly conservative tracer
(Broecker).
8.3
Dissolved silica
- non-conservative. In seawater it is present as H2SiO4
(silicic acid) rather than silicate (SiO3), but many people
use the term silicate. This nutrient is also depleted in
surface waters similarly to nitrate and phosphate -
completely depleted in downwelling areas and small but
measurable quantities in upwelling areas. Subsurface
distributions of silica look something like nitrate and
phosphate and mirror oxygen since silica is also regenerated
in situ below the euphotic zone. However, silica in marine
organisms is associated with skeletons rather than fleshy
parts and so dissolves more slowly in the water. Much of the
silica thus falls to the bottom of the ocean and accumulates
in the sediments. Dissolution from the bottom sediments
constitutes a source of silica for the water column which is
not available for nitrate, phosphate or oxygen. Another
independent source of silica are the hydrothermal vents
which spew water of extremely high temperature, silica
content, and helium content, as well as many other minerals,
into the ocean. These three quantities are used commonly to
trace hydrothermal water.
Property-property
relations (O2/NO3, O2/PO4, NO3/PO4, O2/SiO4) (umol/kg)
Other
Resources for property sections
Hydrographic
Atlases from the World Ocean Circulation Experiment
during the 1990s
Ocean
Data View

Updated
on Sep 2014
Lecture
5: Property Distributions, water masses, and tracers.
Link
to Lecture 5 slide show: 5_watermassdistribution.pdf
The
distribution of water masses and their properties around the
global ocean, including typical property profiles, and
examples of meridional and zonal sections of water properties
in the Atlantic, Indian, and Pacific Oceans. Identifying water
masses on a T/S diagram. Conservative, non-conservative, and
transient tracers.